By Farhad Boltchi, DMD, MS
The sinus augmentation/sinus lift procedure has become an integral and predictable aspect of surgical implant therapy. Traditionally the lateral window sinus augmentation procedure has been regarded as the standard technique to perform this procedure. Although this is a highly successful and predictable technique it is also more invasive and associated with a higher morbidity. The transcrestal sinus lift procedure was developed as a less invasive but still an equally predictable alternative technique for the augmentation of the maxillary sinus. However, this approach is typically limited to simultaneous implant placement and sinus lift scenarios where a minimum residual bone height of 4mm is present. In cases where the residual bone height to the maxillary sinus is less than 3-4mm the lateral window sinus augmentation technique is still regarded as the gold standard. Here we present an alternative minimally invasive staged transcrestal sinus lift technique for cases with minimal residual bone height.
A healthy female patient presented with a missing tooth site #3. Clinical examination revealed a moderate bucco-lingual deficiency and radiographic examination revealed a moderate buccal bone deficiency and a severe vertical bone deficiency due to a significantly pneumatized maxillary sinus (Figure 1). The residual bone height to the maxillary sinus was 1-2mm. The patient was informed of the need for a staged maxillary sinus augmentation/sinus lift procedure to be followed by implant placement 6-7 months later. A surgical treatment plan was devised to attempt the staged sinus lift procedure via a transcrestal approach initially and only resort to a lateral window approach if the transcrestal approach would result in a perforation of the Schneiderian sinus membrane rendering bone graft containment unsuccessful and thus necessitating a lateral window approach to repair the membrane perforation and perform the sinus augmentation procedure. The surgical steps of the staged transcrestal sinus lift procedure were as follows:
1.) Preparation of a full thickness bucco-lingual envelope flap sites #2-4.
2.) Prepare an osteotomy 1mm short of the sinus floor with the final drill diameter (in this case 3.7mm) corresponding to the anticipated implant to be placed (in this case a 4.2mm implant). This step can be performed guided as well.
3.) Use an end cutting transcrestal sinus lift bur with stoppers (Meisinger Crestal Lift Control Kit) to access the sinus floor and cut through the cortical floor of the sinus without perforating the Schneiderian membrane. In this case the 3.8mm Meisinger Crestal Lift Control bur was used.
4.) Prepare the bone graft material. In this case a mixture of a xenograft (Bio-Oss, Geistlich Pharma) and platelet-rich-fibrin (PRF) was utilized.
5.) Insert the bone graft mixture into the osteotomy in increments and lift the Schneiderian sinus membrane via the bone graft mixture with the Densah osseodensification technique (Versah, LLC). In this case the Densah VT3545 bur was used. It is important to perform this step with the Densah bur rotating in the reverse osseodensification mode at 50 RPM and without irrigation. It is also important not to extend the bur more than 1mm into the sinus. In this case this step was performed 10-11 times in increments to slowly lift the sinus Schneiderian membrane approximately 10mm (Figs. 2-3).
6.) Augmentation of the buccal deficiency with a mixture of allograft bone (Maxxeus Cortical Min/Demin Blend) mixed with PRF and overlayered with a long-resorbing membrane (Pericardium).
7.) Primary closure of the surgical flaps with non-resorbable sutures (5.0 Polypropelene).
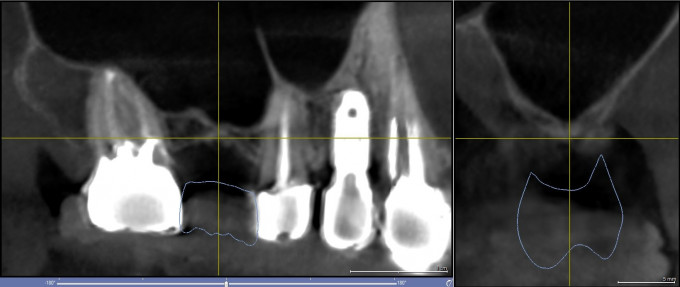
Figure 1: Pre-operative CBCT scan images of site #3
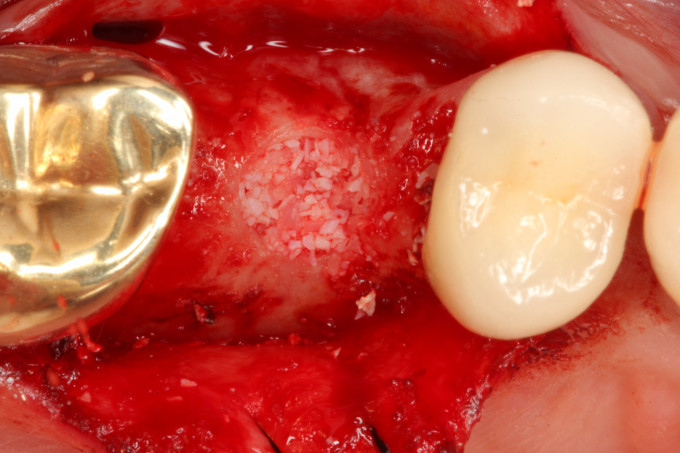
Figure 2: Occlusal view after completion of the staged transcrestal sinus lift procedure
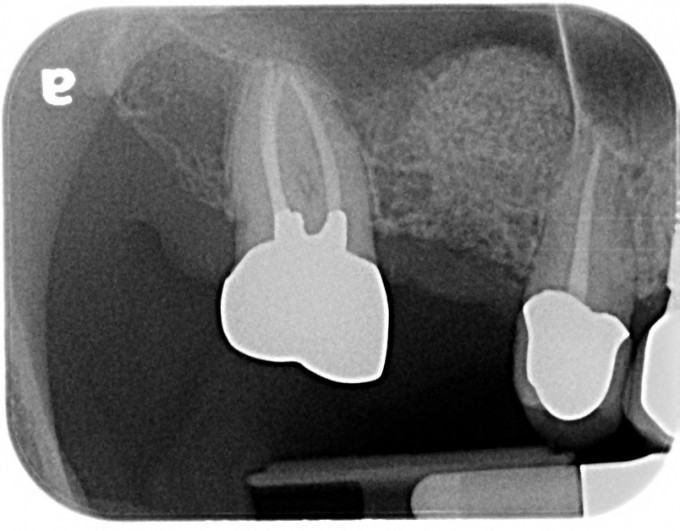
Figure 3: Periapical radiograph of the staged transcrestal sinus lift procedure
After an uneventful healing period of 6 months a periapical and cone beam CT radiographic evaluation was performed and implant planning was carried out in the SICAT 2.0 implant planning software (Figure 4). The digital implant plan was uploaded to SiCat in Germany for design of a Digital Guide, which was 3D printed in-house. The surgical steps of the second stage surgery were as follows:
1.) Preparation of a full thickness bucco-lingual envelope flap sites #2-4.
2.) Fully guided placement of an Astra EV S 4.2 X 9mm implant (Figure 5).
3.) Minor additional buccal augmentation with a mixture of a xenograft (Bio-Oss, Geistlich Pharma) and platelet-rich-fibrin (PRF) overlayered with a long-resorbing ossifying collagen membrane (Ossix Volumax, Dentsply Sirona) and with a PRF membrane.
4.) Placement of a transmucosal healing cap (Astra EV Healdesign 5 X 4.5) and non-submerged suturing around the healing cap with a non-resorbable suture (5.0 Cytoplast, Osteogenics).
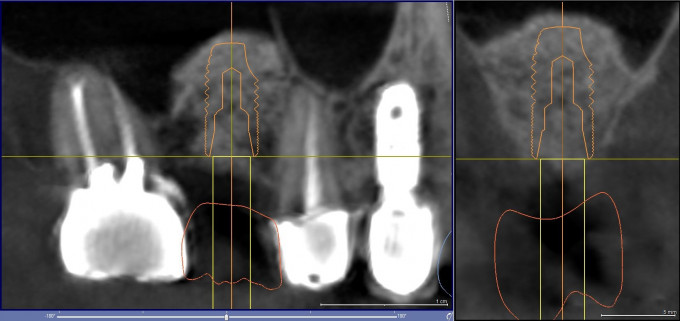
Figure 4: Second stage implant plan in SICAT 2.0
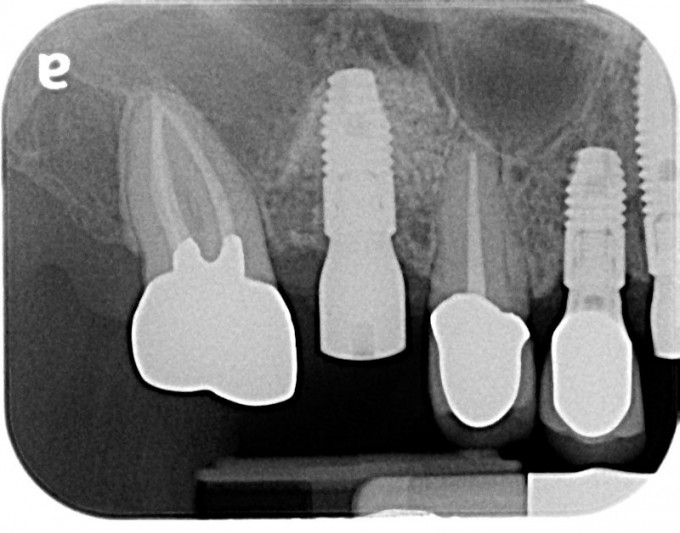
Figure 5: Immediate post implant placement periapical radiograph
After an additional uneventful healing period of 3 months an Atlantis IO FLO ScanBody was inserted into the implant and a CEREC Primescan digital impression of the implant was obtained and uploaded to Atlantis. Atlantis designed and fabricated a titanium custom base and the corresponding e.max crown was milled in-house from the core file provided by Atlantis for a final screw-retained implant crown (Figs. 6-8).
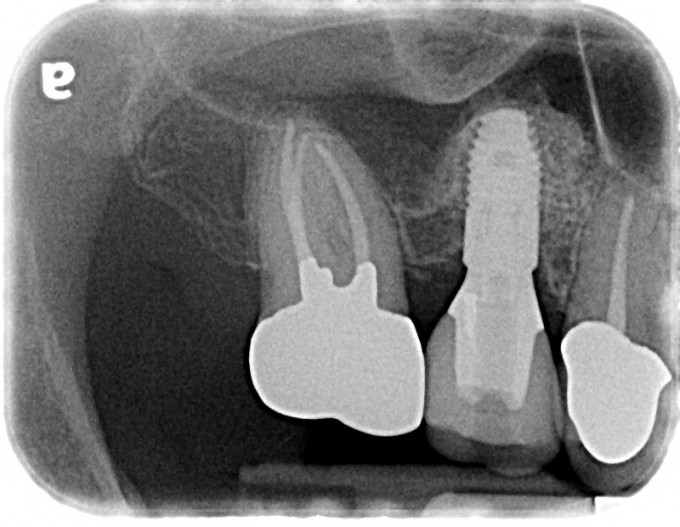
Figure 6: Final periapical radiograph of restored implant
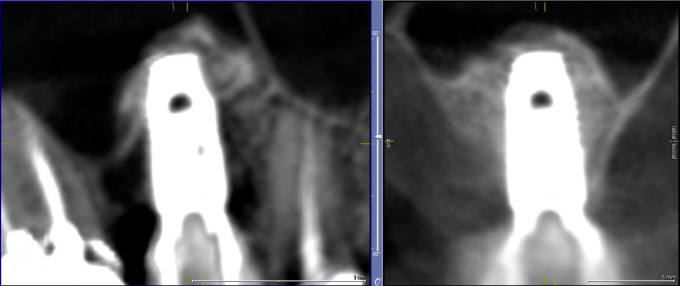
Figure 7: Final post-operative CBCT scan of restored implant
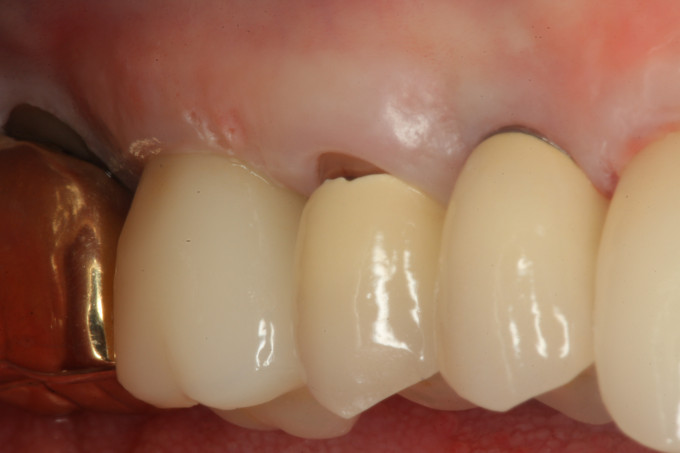
Figure 8: Final clinical view of the restored implant
The staged transcrestal sinus lift procedure provides an attractive minimally invasive alternative to the traditional lateral window sinus lift technique. The key to successful implementation of this technique is to maintain the integrity of the Schneiderian membrane to ensure containment of the bone graft. The surgeon must be prepared to resort to a traditional lateral window sinus lift procedure should a perforation of the membrane occur to repair the membrane perforation and augment the sinus at the same time.
 Farhad Boltchi, D.M.D.
Farhad Boltchi, D.M.D.